
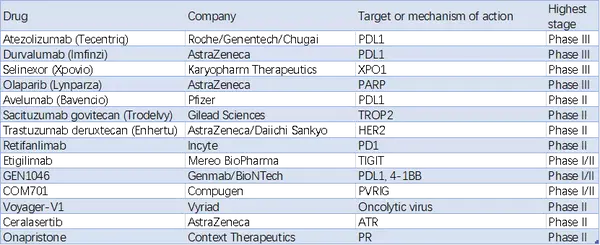
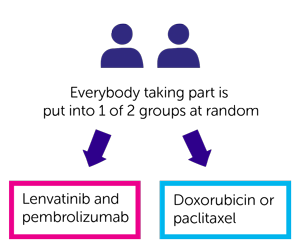
Pembrolizumab at 200 mg intravenously every 3 weeks for up to 35 cycles in combination with lenvatinib at 20 mg orally once daily) lenvatinib could be continued beyond this point if the patient was deriving a benefit.Patients were randomly assigned 1:1 to receive either: Of the 827 patients, 697 patients had tumors that were pMMR, and 130 patients had tumors that were dMMR. Patients could have received two regimens if one was given in the neoadjuvant or adjuvant setting. The study enrolled 827 patients with advanced, metastatic or recurrent endometrial cancer that had experienced disease progression after one prior platinum-based regimen. Food and Drug Administration’s 2019 accelerated approval of the combination in patients with advanced endometrial carcinoma that is not dMMR or microsatellite instability–high (MSI-H), who have disease progression following systemic therapy, and are not candidates for curative surgery or radiation therapy.
KEYNOTE 775 TRIAL
KEYNOTE-775/Study 309 is the confirmatory trial for KEYNOTE-146/Study 111, which supported the U.S. “Lenvatinib/pembrolizumab showed a statistically significant and clinically meaningful improvement in overall survival, progression-free survival, and objective response rate vs treatment by physician’s choice, regardless of MMR status, in endometrial cancer patients progressing after prior platinum-based therapy,” commented Dr. It also met the secondary endpoint of objective response rate in the all-comer population (which included patients with mismatch repair–proficient and mismatch repair–deficient tumors) and in the pMMR subgroup, reported Vicky Makker, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York, at the Society of Gynecologic Oncology (SGO) 2021 Annual Meeting on Women’s Cancer, held virtually. The randomized study of 827 patients met the dual primary endpoints assessed by blinded independent central review-progression-free survival and overall survival-as compared with physician’s choice of treatment (doxorubicin or paclitaxel).


 0 kommentar(er)
0 kommentar(er)
